First this article will describe the condition known as Hydrogen Embrittlement. Further below it will get into how and why Hydrogen Embrittlement is an important factor when Galvanizing any steel or alloy product.
Hydrogen embrittlement occurs in steel when individual hydrogen atoms enter voids in the steel and combine with each other to form hydrogen molecules. The formation of these molecules creates outward pressure. If the product involved is an anchor bolt this pressure can reduce the pull strength of the steel. (fig 1)
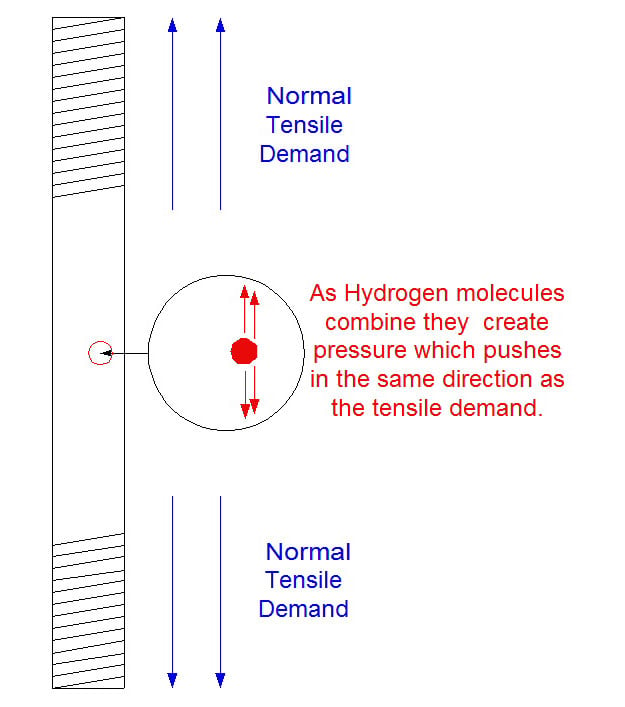
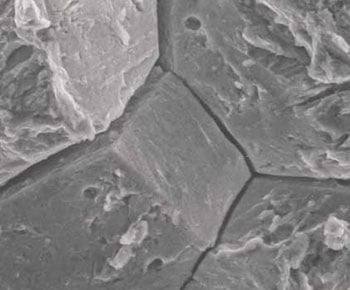
The normal voids mentioned above tend to run a long the grain lines in the steel. That would be where the hydrogen molecules accumulate. If a crack should occur, it will usually follow the contour of the grain line also. In the photo below, which is magnified 1500 times, the cracks that occured are clearly visible.
 While there can be several causes, to the structural steel fabricator the significance of Hydrogen Embrittlement becomes most relevant during the Hot Dip Galvanizing process. Prior to being immersed in molten zinc the steel is prepared in a series of acid baths known as Pickling. These acids contain hydrogen atoms that can migrate into the steel and begin the process described above. The heat of the actual galvanizing process can also be a factor in the process.
While there can be several causes, to the structural steel fabricator the significance of Hydrogen Embrittlement becomes most relevant during the Hot Dip Galvanizing process. Prior to being immersed in molten zinc the steel is prepared in a series of acid baths known as Pickling. These acids contain hydrogen atoms that can migrate into the steel and begin the process described above. The heat of the actual galvanizing process can also be a factor in the process.
As it applies to Anchor Bolts this condition is generally not a factor in standard grades of round bar. Hydrogen Embrittlement becomes a factor when galvanizing higher strength steel, with tensile strength in excess of 150 ksi, such as A-490. ASTM A-143 states Hydrogen Embrittlement becomes a concern when the tesile strength of the steel exceeds approximately 150ksi. Of the three grades of round bar spelled out in the ASTM Anchor Bolt specifications (F1554) only the strongest, Grade A-105 approaches a tensile strength of 150 ksi.